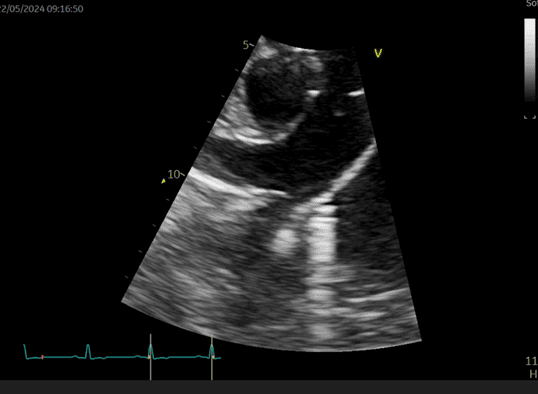
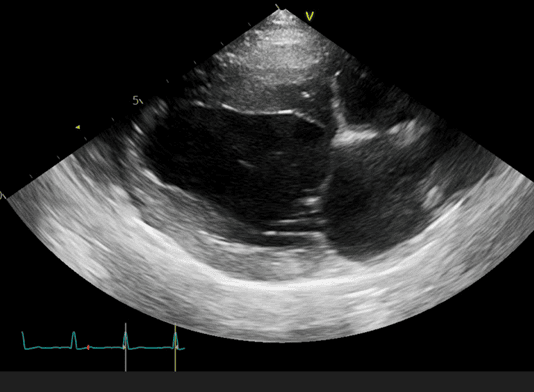
Figure 1 shows Willow’s PDA which is shunting blood from her aorta to pulmonary artery (PA). The pressure within the aorta is much higher than the PA during both systole and diastole, which accounts for the continuous murmur in these patients. The left-to-right shunt results in over-circulation of the lungs and the left-sided heart chambers and figure 2 shows this subtle enlargement of Willow’s left atrium and left ventricle.
The gold standard approach to these cases is PDA closure using a minimally invasive transvascular approach. This involves making a small peripheral incision in the hind limb and accessing the heart via the femoral artery or vein. Plugging the PDA in this manner is far less invasive than a thoracotomy and ligation and is associated with lower morbidity and mortality.
We accessed Willow’s aorta via her femoral artery and performed an angiogram. Figure 3 shows a still image from the angiogram (we can see the pigtail catheter within her aortic root that is used to deliver the contrast). In this frame, the ductus is highlighted (yellow arrows). In most cases, we would close the PDA with a device called an Amplatz canine ductal occluder (ACDO). However, Willow’s ductus had a slightly unusual morphology (quite a large ductus but tapering to an unusually narrow minimal ductal diameter (red arrow)). So instead, we used a device called an Amplatzer vascular plug (AVP-II). The AVP-II has two flat lateral retention discs at either end and a central component in between. Figure 4 shows the AVP-II plugging Willow’s PDA securely, with the distal retention disc deployed within Willow’s pulmonary artery and the central component and proximal retention disc within the ductus arteriosus (which is obviously no longer patent!).


The ability to use either an ACDO or an AVP-II allows us to manage a wider population of patients. Unlike the ACDO, the AVP-II permits a transvenous approach rather than exclusively transarterial, meaning it can be used for very tiny patients whose arteries are not large enough to accommodate a delivery catheter. It is also more suitable for patients like Willow that have an atypical PDA morphology. Sometimes, the morphology and size of the PDA is unclear until the angiogram has been performed in theatre, so being able to make quick decisions on surgical kit in real time gives us more options, and ultimately improves patient safety and outcomes.
Now that her shunt had been closed, Willow’s murmur immediately disappeared. She was discharged the following day and is doing well at home. Willow was lucky in that her PDA had not caused her any clinical problems in her 2 years but she was starting to experience volume overload. Now that her PDA has been successfully closed, there is no reason why Willow should not have a completely normal life and life expectancy.




